Regulatory Officer
Digital Nuclear Diagnostics, Inc.
Job Description
Benefits
Allowances
Meal Allowance, Transportation Allowance
Employee Recognition and Rewards
Performance Bonus, Employee of the Month Award
Government Mandated Benefits
13th Month Pay, Employee Loan, Pag-Ibig Fund, Paid Holidays, Philhealth, SSS/GSIS
Insurance Health & Wellness
HMO
Others
Mobile Phone Discount
Perks Benefits
Training Subsidy
Professional Development
Job Training, Professional Development
Time Off & Leave
Bereavement Leave, Maternity & Paternity Leave, Sick Leave, Solo Parent Leave, Vacation Leave
- Regulatory Officer ensures the appropriate licensing, and legal compliance of pharmaceutical and medical products, in order to control the safety and efficacy of products.
1. Oversees compliance with Food and Drug Administration by submitting the following applications:
- Initial and renewal product registration
- Product variations
- Initial and renewal application for License to Operate
2. Liaises with Food and Drug Administration and other government agencies to facilitate review and approval of applications in a timely manner.
3. Coordinates regulatory submission requirements with the company.
4. Ensures company’s compliance with applicable FDA regulations.
5. Provide regulatory support to Finance, Engineering, and Sales Department.
6. Serves as regulatory affairs support for Pharmacovigilance audit, FDA inspections and internal compliance audit.
7. Attends external seminars and educational sessions to monitor new regulatory updates and to communicate such changes to the President.
8. New Product/s
- Prepares a checklist of requirements based on regulatory agency/ies’ guidelines and requirements
- Collects documentary requirement from Manufacturer/Principal
- Prepares application form/s and necessary attachment/s
- Files application in local regulatory agency
- Upon approval, regulatory officer updates the President
- Safekeeping of the original copies of licenses to proper handling
9. Product/s for renewal registration
- Prepares a checklist of requirements based on regulatory agency/ies’ guidelines and requirements
- Collects documentary requirement from Manufacturer/Principal
- Prepares application form/s and necessary attachment/s
- Files application in local regulatory agency
- Monitors status of application
- Upon approval, updates the President
10. Government mandated registrations for DND operations and for eligibility in joining government biddings
- Prepares a checklist of requirements based on regulatory agency/ies’ guidelines and requirements and monitor the due dates of every permit such as BIR Permits, Business Permits, Mayor’s Permit, Tax Clearance, BOC etc.
- Collects documentary requirement
- Prepares application form/s and necessary attachment/s
- Files application in local regulatory agency
- Monitors status of application
- Upon approval, updates the President
11. Perform other duties as may be assigned by the President
- Monitors status of application
- Graduate of Bachelor of Science in Pharmacy.
- Registered Pharmacist (RPh), with unexpired PRC ID.
- Excellent organizational and planning skills.
- Strong sense of responsibility. Must work with minimal supervision and direction, resourceful, and can work with under pressure.
- Above average computer skills.
- Good communication. Ability to multi-task, organized, attention to details and accuracy.
- Candidate must be willing to work at Greenhills, San Juan City.
- Dynamic fresh graduates are welcome to apply.
- 1-3 work experience
Paul Anthony Samson
Admin ManagerDigital Nuclear Diagnostics, Inc.
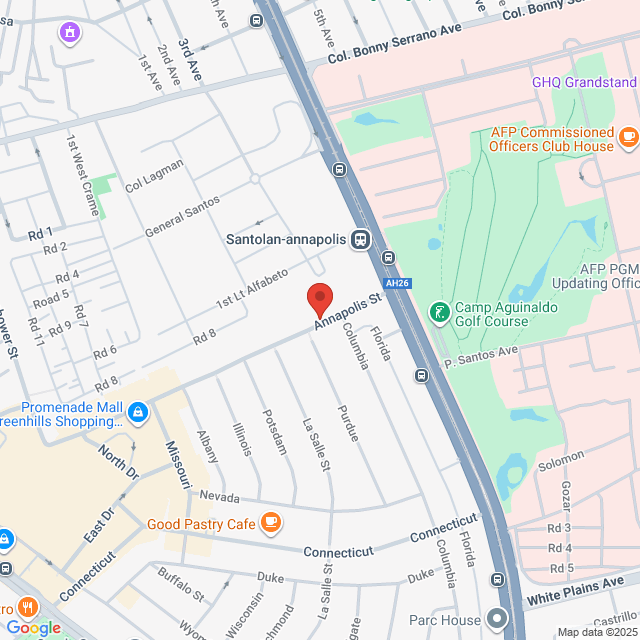
Working Location
UNIT 1501, Unit 1501, Annapolis Wilshire Plaza, 11 Annapolis St, San Juan City, Metro Manila, Philippines
Posted on 04 September 2025
Explore similar jobs
View more similar jobsRegulatory Officer - Open for Fresh Grad - Reg Pharmacist - Greenhills, San J...
 Dempsey Resource Management Inc.
Dempsey Resource Management Inc.Дин34.4-43K[Monthly]
On-site - San JuanFresh Graduate/StudentBachelorFull-time
Aisa Refugia
Regulatory Staff
 Intramuros Holdings Corporation
Intramuros Holdings CorporationДин25.8-34.4K[Monthly]
On-site - MakatiFresh Graduate/StudentBachelorFull-time

Patricia DHR Officer
Pollution Control Officer
 TKL Steel Corporation
TKL Steel CorporationДин34.4-43K[Monthly]
On-site - Manila3-5 Yrs ExpBachelorFull-time

TKL STEEL CORP HRDHR Officer
Regulatory Affairs Officer
 Medtecs International Corp. Ltd. - Philippines
Medtecs International Corp. Ltd. - PhilippinesДин43-51.6K[Monthly]
On-site - Makati1-3 Yrs ExpBachelorFull-time
jean dayagHR Supervisor
Regulatory Officer
 Keiko Food Corp.
Keiko Food Corp.Дин43-60.2K[Monthly]
On-site - Taguig1-3 Yrs ExpBachelorFull-time

Regina SilangHR Manager

Digital Nuclear Diagnostics, Inc.
<50 Employees
Retail Trade
View jobs hiring
Sign In to Chat with Boss
Bossjob Safety Reminder
If the position requires you to work overseas, please be vigilant and beware of fraud.
If you encounter an employer who has the following actions during your job search, please report it immediately
- withholds your ID,
- requires you to provide a guarantee or collects property,
- forces you to invest or raise funds,
- collects illicit benefits,
- or other illegal situations.